Back to top
The cell membrane separates the intracellular and extracellular solution. The sites differ in the compositions of dissolved electrolytes, both have the same concentration of osmotic active particles of 300 mmol/litre and both are net electroneutral (the number of positive charges is the same as negative charges).
There are more potassium ions inside the cell and more sodium and the chlorine ions outside. At rest, the membrane is permeable for several different ions, most notably potassium. In general, the diffusion translocates an ion from the area with the higher concentration to the area with the lower, which means out of the cell in the case of potassium ions. Difference in the concentrations of the ion results in the chemical potencial. Along each potassium ion, one positive charge is transferred to the outside, resulting in excess negative charge inside the cell. Electrical potencial depends on the charge of the ion. The electrical and diffusional forces that influence movement of an ion across the membrane jointly form its electrochemical gradient - the gradient of potential energy that determines in which direction ion will flow spontaneously. The net flow of ions is zero, when the electrochemical potentials on both sides of the membrane equalize or - in other words - when the difference in electrical potential energy is conversely equal to the difference in chemical potential energy. Nernst potential or equilibrium potential is the equilibrium value of the electric potential, at which the transition stops.
In reality, the membrane is permeable to several ions, each contributing to the net value of the membrane potential. The extend of the individual ion contribution to the value of the resting potential is determined by its membrane conductivity. The established membrane potential is not equal to the Nernst
potential of any of the ions. Ions are
thus not in their equilibrium state forcing small current. Na + / K + ATPase and Ca2 + ATPase create
currents that
oppose these "small currents" of individual ions. In the long run, the pumps ensure that the net flux of
each ion is
zero and that consequently the ion concentrations on each side of the membrane remain unchanged.
Electrical potential establisched across the membrane at rest is called the
resting membrane potential (MMP)
.
If we recall the basics of electrical engineering, we can draw many parallels between the membrane and the
electrical
circuit with a capacitor.
The smooth operation of the system at a higher level requires the coordination and organization of the building blocks of the lower level - so the different parts of the cell or cells communicate with each other, namely through electrical and/or chemical signals. Electrical communication between cells takes place by spreading a change in membrane potential. Chemical communication takes place through the secretion of neurotransmitters, hormones or cytokines.
Various stimuli (mechanical, chemical, light, electrical) trigger the flow of ions across the plasmalemma, triggering a change in the resting potential (MP). The change in potential caused by the action of a neurotransmitter is called the postsynaptic potential (PSP). However, when the change in MP in the sensory nerve endings is triggered by an external stimulus, the potential is called the receptor potential.
The flow of positive ions into the cell (as part of signal propagation) or the flow of negative ions out depolarizes the membrane. It means that the conductivity of the membrane for sodium ions increases extremely quickly and "exceeds" the resting conductivity for potassium ions. Membrane potential becomes less negative or even reaches positive values. In the repolarization phase - when the signal is complete - the membrane is repolarized due to two mechanisms; the Na + channels are inactivated soon after depolarization, which means that they close with a delay and prevent further entry of sodium ions. Another process is the increase of the membrane conductivity for potassium ions. Flow of potassium ions is slower than sodium one. Both are activated by the same signal – depolarisation. The membrane potential becomes more negative in the repolarization phase.
A few words about postsynaptic potential; Contacts between neurons or between neurons and target cells are called synapses. On one side are the axonal ends of the presynaptic neuron and on the other side are the dendrites or body (also called soma, perikaryon) of the postsynaptic neuron. When the action potential (AP) reaches the terminal part of the first neuron, a neurotransmitter is released from the tips and binds to a receptor on the membrane of the second neuron. Depending on the neurotransmitter and the postsynaptic cell response, we divide postsynaptic potentials into excitatory (EPSP) - the neurotransmitter triggers depolarization of the postsynaptic membrane and stimulates certain activity in the target cell - or inhibitory (IPSP) - triggers hyperpolarization and reduction of the negative membrane value. The magnitude of the postsynaptic potential varies as it depends on the individual EPSPs, IPSPs.
Neuron can receive multiple impulses at the same time. Spatial summation is the summation of excitatory postsynaptic potentials (EPSPs) that travel through different dendrites of a nerve cell in the same time interval. Thus, EPSP is larger than that which comes from only one synapse. The time summation occurs when several EPSPs come in succession at short intervals, in which the previous EPSP does not settle down when a new one arrives. This adds its amplitude to the already existing amplitude of the previous EPSP, which triggers off several action potentials.
A larger stimulus causes a greater change in membrane voltage, therefore we designate PSP and receptor potential as graded potentials. For example: higher light intensity causes greater hyperpolarization; more Ach causes greater depolarization in neuromuscular junction. Because we do not have fast Na + voltage-dependent channels in the dendrites and body of the neuron, AP cannot be triggered here. The signal propagates electrotonically. It is a redistribution of the charge that has come into the cell in the area of the synapse. Propagation is mainly influenced by the passive properties of the nerve cell membrane; time and length constant .
The potential decreases
with distance from the place of origin.

Many subthreshold potentials are transmitted electrotonically forward over short distances. The electrical impulse therefore dies at a short distance, and does not reach the end of the axon of a large neuron. The membranes of the excitable cells have adapted their membranes
so that the electrical impulse is "capable" of traveling the entire length of the
long axon and the
potential strength is not (significantly) reduced. Sufficient depolarization can cause transient occurrence,
so called action
potential. The action potential is triggered when the depolarization reaches a threshold
value. The
threshold value is the MP
value at which there is more than a 50 % probability that AP will occur. Receptor potential either triggers
or does not trigger
AP - on the principle of all or nothing. Depolarization of the membrane to the threshold value
activates
Na+ voltage-dependent
channels and increases the conductivity of the membrane for sodium.
To a lesser extent, the conductivity
also increases for
calcium. The energy that allows the triggering of AP is stored in the transmembrane ion concentration
gradient and is released
with increasing
ion channel permeability;
sodium and calcium ions consequently enter the cell, triggering a
change in the
polarization of the plasmalemma. Because Nernst’s sodium and calcium potentials are very positive, the MP
membrane becomes very
positive. To keep the ion concentrations unchanged in the long run, both outside and inside the cell, the
pumps ensure that they
temporarily increase their activity during and after the AP. Before the ion concentrations on both sides
change significantly,
more than 100 APs can be triggered.
As a local anesthetic lidocaine prevents the formation and transmission of nerve impulses in the sensory,
motor and autonomic nerves. It acts mainly on the cell membrane, where it blocks ion channels and thus
reduces the permeability of sodium ions. Due to the progressive spread of the anesthetic effect in the
nerve, the threshold of electrical excitability is raised, the conduction of impulses is slowed down, and
the spread of the action potential is shortened; in the end, the conductivity is ended.
When the signal reaches the axon hillock i.e. the region of the neuron where the density of fast Na + voltage-dependent channels is high, AP can form. Whether the potential change for AP formation will be large enough, depends on the sum of all the effects from different synapses from different neurons on the postsynaptic neuron — that is, all EPSP and IPSP. When the sum rises even more, the AP frequency increases. In this way, the amplitude of the membrane potential is recoded into the frequency of the action potentials.
a) AP DOES NOT OCCUR
If the depolarization does not reach the stimulus threshold locally, AP is not provoked at this point. The change of potential depends on the distance, because the magnitude of the change decreases exponentially from the place of origin - the propagation is electrotonic/passive. The changes therefore do not spread far along the axon.
b) AP OCCURS
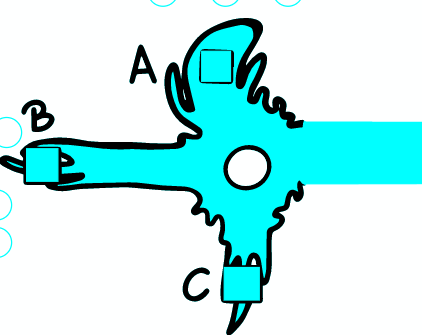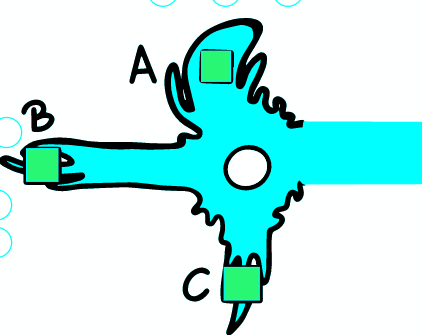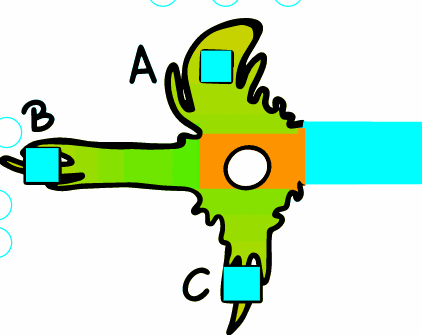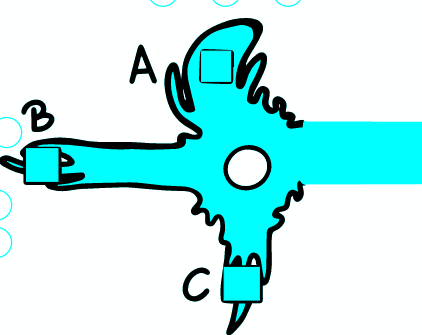
The basic function of axons is to transfer impulses over longer distances. To illustrate, the axon of the motor nerve is more than 1 meter long. The AP propagates along the entire length of the axon without signal decay. The shape and amplitude of the AP are also constant.
During AP travel, rapid depolarization ensures a sufficiently large flow of ions into the cell. This causes a local change in the MP that propagates electrotonically forward. Local depolarization currents activate Na + voltage-dependent channels and thus depolarize the adjacent part of the membrane. If the threshold is exceeded locally, AP is triggered at this point. The membrane through which the AP has passed becomes transiently refractory, insensitive to new APs. This allows one-way AP translation along the fiber .

Smaller nerve fiber

Bigger nerve fiber

Bigger nerve fiber with myelin sheaths
Nerve fibers are classified according to the rate of conduction, which depends as said, on the size
of the fiber and the
presence of myelin sheaths. The larger the fiber diameter is and the presence of myelin sheaths the
faster the translation
will be. There are two classification systems based on differences in translation speed; The
Erlanger and Gasser
classification refers to sensory (afferent) and motor (efferent) nerve fibers and uses nomenclature
A, B, C. Lloyd and
Hunt classification refers only to sensory fibers and uses numerical nomenclature; I, II, III, IV.
The table lists the individual fiber types, their diameter, conduction velocity, and the presence of
myelin sheaths.
hide
At every 1-2 mm, a 1 qm wide gap in the myelin sheath has the node of Ranvier. Therefore, only
individual
sections
of the axon are activated where AP formation occurs, as the majority of Na + channels are located here. The
impulse thus jumps
from segment to segment - saltatory translation. The distance where the potential dies is longer than
the
distance between
two nodes. The myelin sheath thus affects that AP is formed every 1-2 mm, in contrast to non-myelinated
axons, where AP is
formed continuously along the entire axon. Saltator translation is thus faster and "not wasteful of
energy",
as Na + / K +
ATPases are found only in the nodes. Speed of the AP travel is determined by longitudinal resistance
(fiber
cross section),
the number of open channels in the membrane at a given time, membrane capacitance, the density of
voltage-sensitive Na +
channels, which determines how much depolarization current will enter a cell in a particular segment of the
fiber and the
myelin
sheath
(accelerates AP translation in several ways: increases the length constant, reduces the time constant,
increases the
amount of current that enters a cell in the nodes of Ranvier).
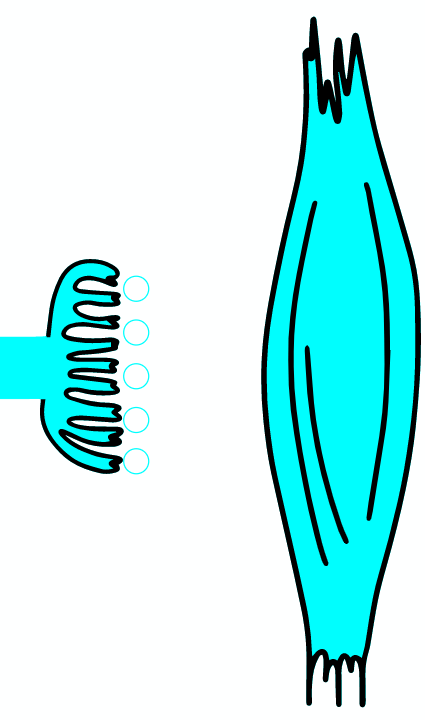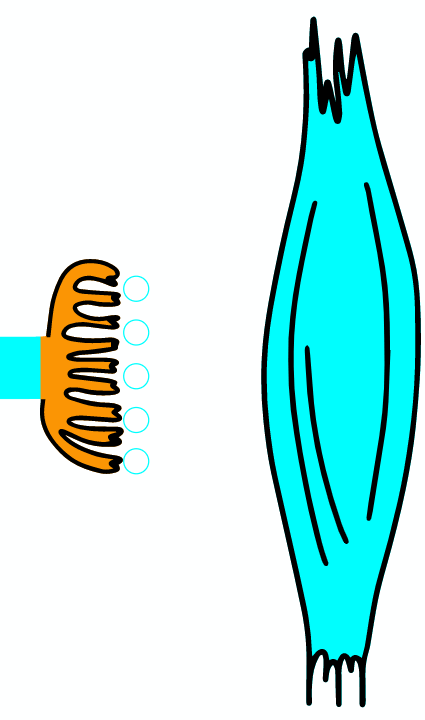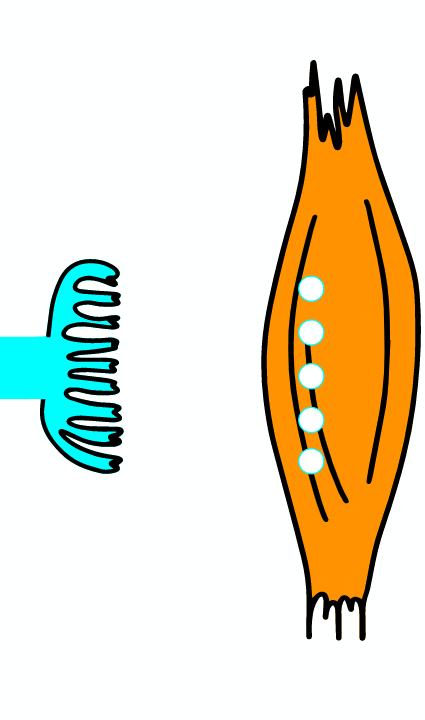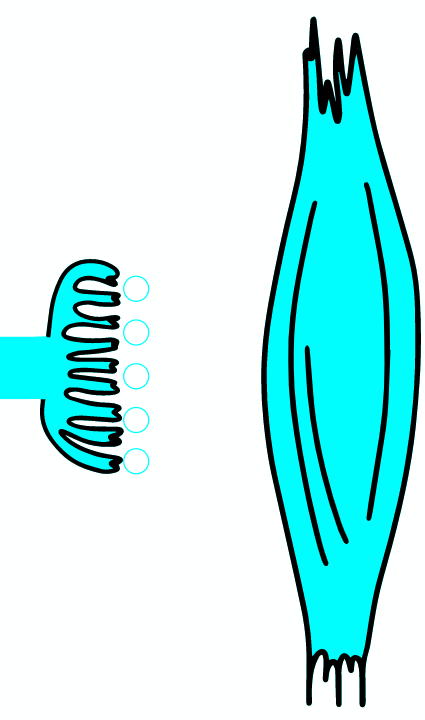
As the AP reaches the end it triggers the opening of Ca2 + voltage-dependent channels. Calcium ions enter the cell and bind to a specific protein synaptotagmin, which acts as a calcium sensor and regulates the closure of the SNARE complex (the complex consists of the proteins synaptobrevin, syntaxin and SNAP-25).
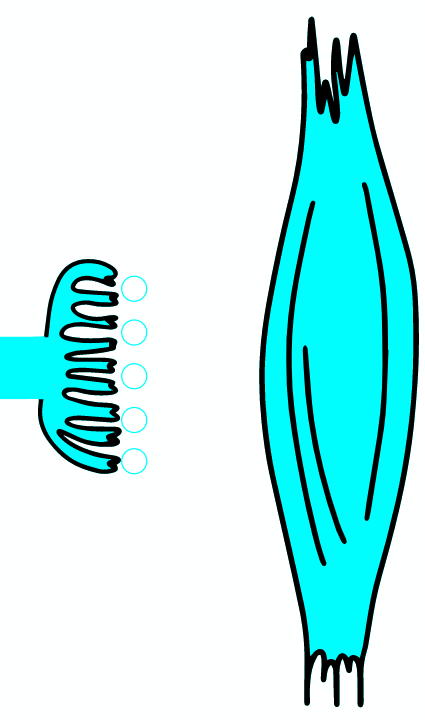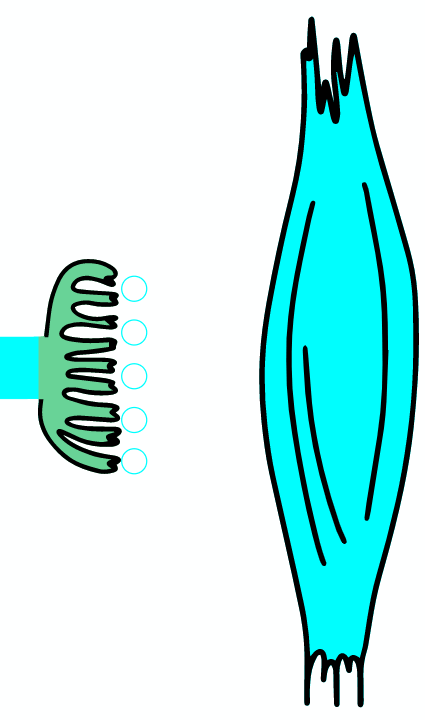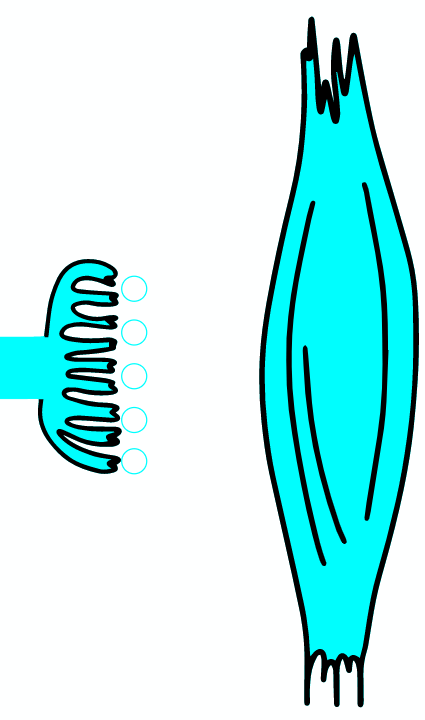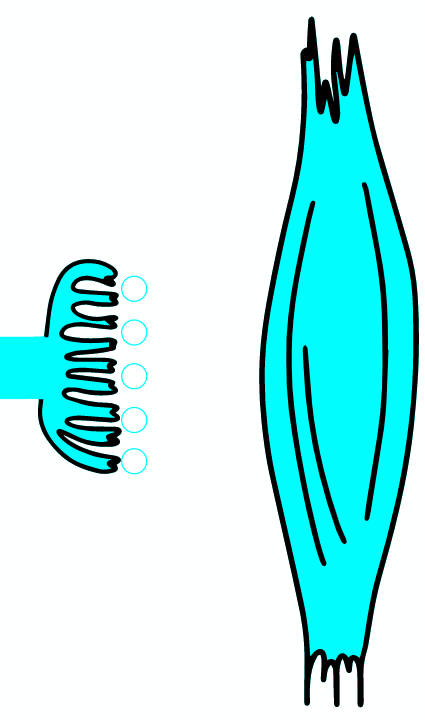
Botulinum toxin is a neurotoxic protein produced by the bacterium Clostridium botulinum and related species. It is the cause of botulism. Humans most commonly ingest the toxin from eating improperly canned foods, in which C. botulinum has grown. Toxin can also be introduced through an infected wound. In infants, the bacteria can sometimes grow in the intestines and produce botulinum toxin within the intestine and can cause a condition known as floppy baby syndrome. Toxin binds specifically to nerves which use the neurotransmitter acetylcholine. Once bound to the nerve terminal, the neuron takes up the toxin into a vesicle by receptor-mediated endocytosis. As the vesicle moves farther into the cell, it acidifies, activating a portion of the toxin which triggers it to push across the vesicle membrane and into the cell cytoplasm. Once inside the cytoplasm, the toxin cleaves SNARE proteins, meaning that the acetylcholine vesicles can't bind to the intracellular cell membrane. Preventing the cell from releasing vesicles of neurotransmitter, stops nerve signaling, leading to flaccid paralysis.
In severe cases, the toxin block nerves controlling the respiratory system or heart, resulting in death. Botulism can be difficult to diagnose, as it may appear similar to diseases such as Guillain–Barré syndrome, myasthenia gravis, and stroke. By acting on the muscles, we can relieve muscle cramps or dystonia in cerebral palsy, it can be used to treat certain migraine headaches, and through the effect on the functioning of the sweat glands, we can also treat excessive sweating or hyperhidrosis, etc. Botulin is used in anesthetic medicine to correct facial expressions and smooth wrinkles on the face and neck. The action of botulinum is limited in time to about 4 months, after which it must be re-administered. It may therefore come as no surprise that: The global market for botulinum toxin products, driven by their cosmetic applications, is forecast to reach $ 2.9 billion by 2018. hide
The role of
synaptotagmin
is to bring
together the lipid
bilayers of the vesicle and the terminal membrane and to induce their fusion.
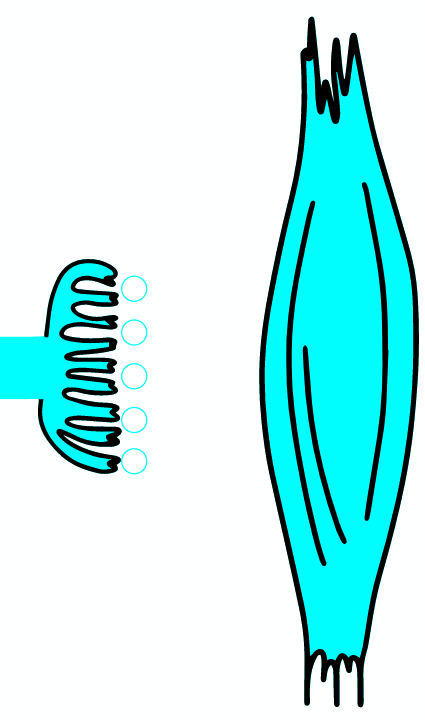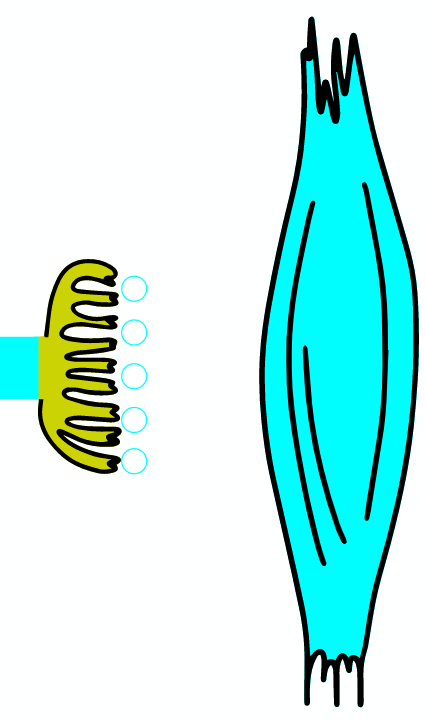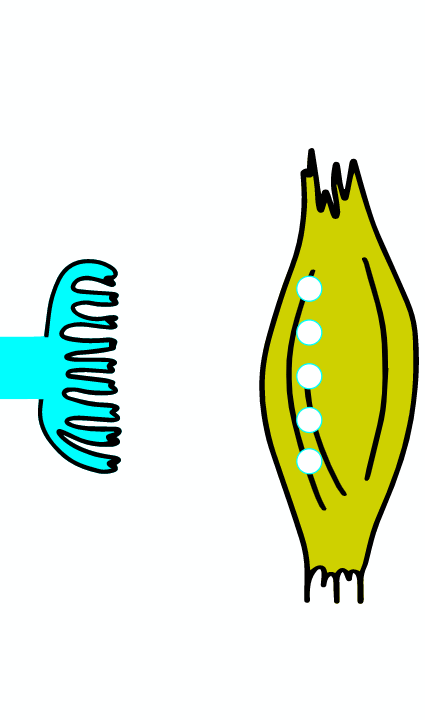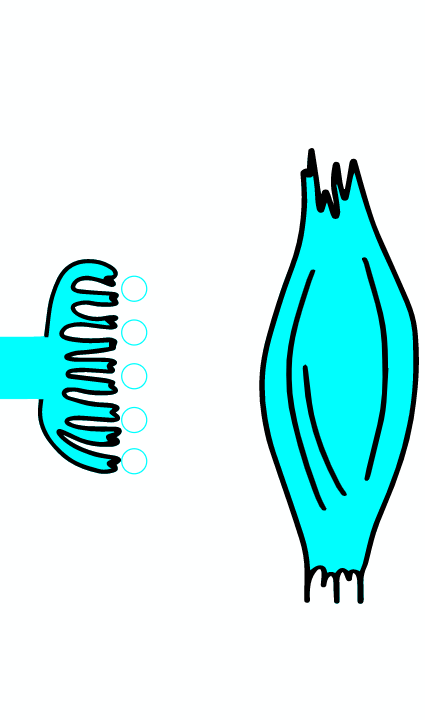
When infected with Clostridium tetani, the tetanus toxin reaches the inhibitory neuron, where it cleaves the synaptobrevin protein from the protein complex, which ensures the fusion of the vesicle with the membrane. Consequently, the fusion of the vesicle containing glycine with the terminal membrane is inhibited. Glycine are not released and cannot pass on the inhibitory effect of the neuron. Because there is no inhibition of contraction, the toxin is also called tetanospasmin, as it causes a characteristic contraction of the body.
The bacteria's toxin reaches the spinal cord via retrograde axonal transport through peripheral nerves or via the blood circulation. Renshaw cells are inhibitory interneurons of the spinal cord that connect to alpha motoneurons of the anterior gray column. They receive input from the descending tracts of supra-spinal origin and the axon collaterals of alpha motoneurons. The neurotransmitter activating Renshaw cells is acetylcholine. Subsequent to their activation, Renshaw cells create an inhibitory postsynaptic potential (IPSP) within the alpha motoneurons by means of inhibitory neurotransmitters glycine and gamma-Aminobutyric acid (GABA). Inhibition prevents overshooting muscle contraction as a result of the muscle's proprioceptive reflex. Alpha motoneurons create a negative feedback loop by causing cholinergic activation of Renshaw cells. This activation of Renshaw cells inhibits the same alpha motoneurons that activated it. This process is called recurrent inhibition. Wound infection with Clostridium tetani causes a pathological disturbance of Renshaw inhibition. Breakdown of Renshaw inhibition first causes hyperreflexia. Later the separate muscular twitches merge into one permanent contraction (spasm) because the alpha motoneurons are activated constantly. This permanent contraction is the reason for the typical signs of tetanus: risus sardonicus, opisthotonus, convulsion. hide
Consequently the
neurotransmitter is released
into the synaptic cleft and diffuse across to reach its receptors on the postsynaptic membrane. There it
triggers a change
in membrane potential, eliciting excitation or inhibition of the postsynaptic neuron. Neurotransmitters are
divided in the
three groups: excitatory, inhibitory and modulatory neurotransmitters. Excitatory neurotransmitters
have
excitatory effects
on the postsynaptic neuron, meaning they increase the likelihood that the neuron will fire an action
potential. Some of
the major
excitatory neurotransmitters include epinephrine and norepinephrine. Inhibitory neurotransmitters
have
inhibitory effects
on the postsynaptic neuron; they decrease the likelihood that the neuron will fire an action potential. Some
of the major
inhibitory
neurotransmitters include serotonin and gamma-aminobutyric acid (GABA). Some neurotransmitters, such as
acetylcholine
and dopamine, can create both excitatory and inhibitory effects depending upon the type of receptors that
are present.
Modulatory neurotransmitters, often referred to as neuromodulators, can affect a larger number of
neurons at
the same
time. These neuromodulators also influence the effects of other chemical messengers. In contrast to synaptic
neurotransmitters, which are released by axon terminals to have a fast-acting impact on other receptor
neurons,
neuromodulators diffuse across a larger area and are more slow-acting.
Motor plate is a chemical synapse between the α-motoneuron and the muscle fiber. AP reaching the presynaptic membrane causes follicular exocytosis with the neurotransmitter acetylcholine (ACh). It diffuses to nicotinic acetylcholine receptors on the postsynaptic membrane. Depolarization in the neuromuscular synapse to the excitatory threshold requires 100 "quantans" or vesicles. One vesicle depolarizes the motor plate by 0.4 mV and is said to contain less than 10 000 ACh molecules. Acetylcholinesterase (AChE) is an enzyme, located on the postsynaptic membrane, that is classified as a hydrolase because it hydrolyzes ACh. Each molecule of this enzyme breaks down 25 000 molecules of acetylcholine in one second. The active site of acetylcholinesterase comprises two subsites, the anionic site and the ester site. A positive quaternary amine from the acetylcholine molecule binds to the anionic site but can also bind cationic substrates and inhibitors. The ester site consists of a catalytic triad of three amino acids, namely serine, histidine, and glutamate. At this point, acetylcholine is hydrolyzed to acetate and choline to form the acetyl enzyme and free choline. The acetyl enzyme is subsequently degraded to a free enzyme capable of reactivation and an acetyl group. The newly formed free choline is transferred back to the nerve, where acetylcholine is re-synthesized from acetyl coenzyme A via the action of acetylcholine transferase. Acetylcholine esterase inhibitors are substances that bind to the molecule of the enzyme acetylcholine esterase and thus inhibit the hydrolysis of the natural substrate, i.e. acetylcholine. Inhibitors are either reversible or irreversible. A typical representative of a reversible AChE inhibitor is decamethonium - a derivative of the decamethonium molecule is the drug donepezil, which is used in the treatment of Alzheimer's disease. Other reversible AChE inhibitors used clinically are edrophonium (a short-acting inhibitor used to diagnose myasthenia gravis), neostigmine (a medium-acting inhibitor used orally to treat myasthenia gravis, and parenterally to interrupt neuromuscular block). A medium-acting inhibitor is used in the form of eye drops to treat cataracts (glaucoma). Irreversible AChE inhibitors include insecticides, pesticides, and chemical warfare poisons such as nerve gas.
And the story goes on…
Signal transmission
The cell membrane separates the intracellular and extracellular solution. The sites differ in the compositions of dissolved electrolytes, both have the same concentration of osmotic active particles of 300 mmol/litre and both are net electroneutral (the number of positive charges is the same as negative charges).
There are more potassium ions inside the cell and more sodium and the chlorine ions outside. At rest, the membrane is permeable for several different ions, most notably potassium. In general, the diffusion translocates an ion from the area with the higher concentration to the area with the lower, which means out of the cell in the case of potassium ions. Difference in the concentrations of the ion results in the chemical potencial. Along each potassium ion, one positive charge is transferred to the outside, resulting in excess negative charge inside the cell. Electrical potencial depends on the charge of the ion. The electrical and diffusional forces that influence movement of an ion across the membrane jointly form its electrochemical gradient - the gradient of potential energy that determines in which direction ion will flow spontaneously. The net flow of ions is zero, when the electrochemical potentials on both sides of the membrane equalize or - in other words - when the difference in electrical potential energy is conversely equal to the difference in chemical potential energy. Nernst potential or equilibrium potential is the equilibrium value of the electric potential, at which the transition stops.
Written above with the equations:
The chemical potential energy of an individual ion (\(µ\)) is the sum of the chemical potential
energy of a particle under standard conditions (\(µ_0\)) and the product, where \(k\) is the
Boltzmann constant, \(T\) temperature in degrees Kelvin and \(\ln c\) the natural logarithm of the
concentration of this ion:
\[μ = μ_0+k T \ln c\]
Electric potential energy (\(ε\)) is the product between the electric potential (\(φ\)), valence
(\(z\)) and elemental charge (\(e\)).
\[Ɛ=φ z e\]
The net transfer of ions stops when the difference in electrical potential energy (inside and
outside the cell) and the difference in chemical potential energy (inside and outside the cell) are
opposite:
\[Ɛ_{IC} - Ɛ_{EC} = -[μ_{IC} - μ_{EC}] \]
If we insert the first two written equations into the latter and point out the difference between
the electric potentials, we get:
\[(φ z e)_{IC}- (φ z e)_{EC} = - [(μ_0+k T \ln c)_{IC} – (μ_0+k T \ln c)_{EC}]\]
\[z e (φ_{IC}- φ_{EC}) = - k T [\ln c_{IC} – \ln c_{EC}]\]
\[φ_{IC}- φ_{EC} = - \frac{k T}{z e} [\ln c_{IC} – \ln c_{EC}]\]
\[N = - \frac{k T}{z e} [\ln c_{IC} – \ln c_{EC}]\]
Using the logarithm difference rule and converting the natural logarithm to decimal, we rearrange
the equation into the form:
\[N = - \frac{k T}{z e \log e}\log \frac{C_{IC}}{ C_{EC}}\]
If we take into account the value of constants, and for the temperature the value T = 310 K, we get:
\[N = - \frac{61,5 mV}{z}\log \frac{C_{IC}}{ C_{EC}}\]
This is the equation for calculating the Nernst potential.
The equation is used to calculate the Nernst potentials for common ions
\((K +, Na +, Ca2 +, Cl-)\), which applies to \( 37 ° C\) conditions.
We insert known values of concentrations and valences into the equation.
And the value of the Nernst potential is easily calculated:
hide
| \(Na^+ \) | \(K^+ \) | \(Ca^{2+} \) | |
|---|---|---|---|
| ion concentration inside the cell [mEq/L] | 15 | 125 | 0,1 |
| ion concentration outside the cell [mEq/L] | 142 | 4,4 | 2,4 |
| Nernst potencial [mV] | 60 | -89 | 134 |
In reality, the membrane is permeable to several ions, each contributing to the net value of the membrane potential. The extend of the individual ion contribution to the value of the resting potential is determined by its membrane conductivity. The established membrane potential is not equal to the Nernst
Walter Hermann Nernst (1864-1941) was a German chemist known for his work in thermodynamics,
physical chemistry, electrochemistry and solid state physics. He studied physics and mathematics
at the universities of Zürich, Berlin, Graz and Würzburg. He wrote a mathematical equation
describing the process, now known as the Nernst equation, which relates the electric potential
of the ions to various properties of the cell. Nernst and his students in Berlin proceeded to
make many important physico-chemical measurements, particularly determinations of specific
heats of solids at very low temperatures and of vapour densities at high temperatures.
For his work in thermochemistry he received the Nobel Prize in Chemistry for 1920.
hide
The membrane acts as a capacitor and provides energy for the operation of various molecular
processes. The Nernst
potential for potassium ions is presented as a battery that transfers charges to the capacitor -
membrane via a resistor,
in our case in the role of ion channels for potassium ions. The battery transfers the charge to the
capacitor and charges
it until it reaches a voltage equal to the rated voltage of the battery. In the case of a membrane,
the Nernst potential
for potassium ions sends them out of the cell until a membrane potential equal to it is established.
Ohm's law assumes the following: If the value of MP is not equal to the Nernst potential, then there is a net flux of potassium ions, defined by the difference between the actual MP and \(N_k\) and the resistance of the membrane to potassium ions (\(R_k\)): \[I_K = \frac{MP - N_K}{R_K}\] Conductivity is defined as the inverse value of resistance: \[I_K = [MP - N_K]p_K\] As already mentioned, the membrane is actually conductive for several ions.
Taking into account the statement, we assemble an analog electronic circuit in which batteries (representing Nernst potentials of ions passing through the membrane) are connected in parallel with resistors (representing ion channels for ions), through which the batteries charge a common capacitor (representing the quiescent potential). Thus, we assume that after a certain time, a voltage equal to the quiescent membrane potential is established on the capacitor. If this voltage on the capacitor does not change, consequently the sum of the currents of the individual ions is equal to zero. Each ion current to/from the capacitor would change the voltage. Each ion is not in its steady state and consequently its current is different from zero. But as much charge as flows in also flows out of the capacitor.
Write down the sum of the flows: \[I_K + I_{Na} + I_{Cl} + ... + I_N = 0 \] Each current of an individual ion is replaced by the Ohm form of the equation for the current written above, and when we expose the MP, we obtain the equation for the MP in the steady state: \[MP = \frac{p_K N_K + p_{Na} N_{Na} + p_{Cl} N_{Cl} + ... + p_n N_n}{\sum p}\] By transforming the above equation, we obtain a form where it is seen that each ion, for which the plasmalemma is permeable, contributes a certain proportion of its Nernst potential to the MP value. The proportions for individual ions are determined by the ratio between the conductivity for this ion and the total conductivity for all ions: \[MP = N_K \frac{ p_K }{\sum p} + N_{Na} \frac{ p_{Na} }{\sum p} + N_{Cl} \frac{ p_{Cl} }{\sum p} + ... + N_{n} \frac{ p_{n} }{\sum p} \] We derived the equation under the assumption that the MP value is constant and the net current on the plasmalemma or from the plasmalemma is zero. However, since there are net flows for individual ions, we call this state - the quasi-stationary state. Anionic pumps - Na + / K + -ATPase and Ca2 + -ATPase - are anchored in the cell membrane, which, by creating opposite currents, "neutralize" non-negligible currents that occur in the quasi-stationary state. In the long run, they thus contribute to the net flows of individual ions being zero and the ion concentrations not changing. Such a steady state is established at the resting membrane potential. The contribution of ion pumps must also be taken into account in the equation for the quiescent potential; to begin with, we consider only sodium-potassium. Since in each cycle it pumps 3 sodium ions out of the cell and 2 potassium ions in the opposite direction - into the cell, we write: \[ I^{ATPaza}_{K} = -\frac{2}{3}I^{ATPaza}_{Na}\] If we insert into it \[I_K = [MP -N_K]p_K\] We get \[[MP -N_K]p_K = -\frac{2}{3}[MP -N_Na]p_Na\] and after rearranging the equation: \[MP = \frac{3 p_K N_K + 2 p_Na N_Na}{3 p_K + 2 p_Na}\] If we take into account in the equation that the resting membrane is 15 times more conductive for potassium than sodium ions, we obtain the most appropriate form of the equation for calculating the resting membrane potential. hide
Ohm's law assumes the following: If the value of MP is not equal to the Nernst potential, then there is a net flux of potassium ions, defined by the difference between the actual MP and \(N_k\) and the resistance of the membrane to potassium ions (\(R_k\)): \[I_K = \frac{MP - N_K}{R_K}\] Conductivity is defined as the inverse value of resistance: \[I_K = [MP - N_K]p_K\] As already mentioned, the membrane is actually conductive for several ions.
Taking into account the statement, we assemble an analog electronic circuit in which batteries (representing Nernst potentials of ions passing through the membrane) are connected in parallel with resistors (representing ion channels for ions), through which the batteries charge a common capacitor (representing the quiescent potential). Thus, we assume that after a certain time, a voltage equal to the quiescent membrane potential is established on the capacitor. If this voltage on the capacitor does not change, consequently the sum of the currents of the individual ions is equal to zero. Each ion current to/from the capacitor would change the voltage. Each ion is not in its steady state and consequently its current is different from zero. But as much charge as flows in also flows out of the capacitor.
Write down the sum of the flows: \[I_K + I_{Na} + I_{Cl} + ... + I_N = 0 \] Each current of an individual ion is replaced by the Ohm form of the equation for the current written above, and when we expose the MP, we obtain the equation for the MP in the steady state: \[MP = \frac{p_K N_K + p_{Na} N_{Na} + p_{Cl} N_{Cl} + ... + p_n N_n}{\sum p}\] By transforming the above equation, we obtain a form where it is seen that each ion, for which the plasmalemma is permeable, contributes a certain proportion of its Nernst potential to the MP value. The proportions for individual ions are determined by the ratio between the conductivity for this ion and the total conductivity for all ions: \[MP = N_K \frac{ p_K }{\sum p} + N_{Na} \frac{ p_{Na} }{\sum p} + N_{Cl} \frac{ p_{Cl} }{\sum p} + ... + N_{n} \frac{ p_{n} }{\sum p} \] We derived the equation under the assumption that the MP value is constant and the net current on the plasmalemma or from the plasmalemma is zero. However, since there are net flows for individual ions, we call this state - the quasi-stationary state. Anionic pumps - Na + / K + -ATPase and Ca2 + -ATPase - are anchored in the cell membrane, which, by creating opposite currents, "neutralize" non-negligible currents that occur in the quasi-stationary state. In the long run, they thus contribute to the net flows of individual ions being zero and the ion concentrations not changing. Such a steady state is established at the resting membrane potential. The contribution of ion pumps must also be taken into account in the equation for the quiescent potential; to begin with, we consider only sodium-potassium. Since in each cycle it pumps 3 sodium ions out of the cell and 2 potassium ions in the opposite direction - into the cell, we write: \[ I^{ATPaza}_{K} = -\frac{2}{3}I^{ATPaza}_{Na}\] If we insert into it \[I_K = [MP -N_K]p_K\] We get \[[MP -N_K]p_K = -\frac{2}{3}[MP -N_Na]p_Na\] and after rearranging the equation: \[MP = \frac{3 p_K N_K + 2 p_Na N_Na}{3 p_K + 2 p_Na}\] If we take into account in the equation that the resting membrane is 15 times more conductive for potassium than sodium ions, we obtain the most appropriate form of the equation for calculating the resting membrane potential. hide
DENDRITES AND BODY

The smooth operation of the system at a higher level requires the coordination and organization of the building blocks of the lower level - so the different parts of the cell or cells communicate with each other, namely through electrical and/or chemical signals. Electrical communication between cells takes place by spreading a change in membrane potential. Chemical communication takes place through the secretion of neurotransmitters, hormones or cytokines.
Various stimuli (mechanical, chemical, light, electrical) trigger the flow of ions across the plasmalemma, triggering a change in the resting potential (MP). The change in potential caused by the action of a neurotransmitter is called the postsynaptic potential (PSP). However, when the change in MP in the sensory nerve endings is triggered by an external stimulus, the potential is called the receptor potential.
The flow of positive ions into the cell (as part of signal propagation) or the flow of negative ions out depolarizes the membrane. It means that the conductivity of the membrane for sodium ions increases extremely quickly and "exceeds" the resting conductivity for potassium ions. Membrane potential becomes less negative or even reaches positive values. In the repolarization phase - when the signal is complete - the membrane is repolarized due to two mechanisms; the Na + channels are inactivated soon after depolarization, which means that they close with a delay and prevent further entry of sodium ions. Another process is the increase of the membrane conductivity for potassium ions. Flow of potassium ions is slower than sodium one. Both are activated by the same signal – depolarisation. The membrane potential becomes more negative in the repolarization phase.
A few words about postsynaptic potential; Contacts between neurons or between neurons and target cells are called synapses. On one side are the axonal ends of the presynaptic neuron and on the other side are the dendrites or body (also called soma, perikaryon) of the postsynaptic neuron. When the action potential (AP) reaches the terminal part of the first neuron, a neurotransmitter is released from the tips and binds to a receptor on the membrane of the second neuron. Depending on the neurotransmitter and the postsynaptic cell response, we divide postsynaptic potentials into excitatory (EPSP) - the neurotransmitter triggers depolarization of the postsynaptic membrane and stimulates certain activity in the target cell - or inhibitory (IPSP) - triggers hyperpolarization and reduction of the negative membrane value. The magnitude of the postsynaptic potential varies as it depends on the individual EPSPs, IPSPs.
Neuron can receive multiple impulses at the same time. Spatial summation is the summation of excitatory postsynaptic potentials (EPSPs) that travel through different dendrites of a nerve cell in the same time interval. Thus, EPSP is larger than that which comes from only one synapse. The time summation occurs when several EPSPs come in succession at short intervals, in which the previous EPSP does not settle down when a new one arrives. This adds its amplitude to the already existing amplitude of the previous EPSP, which triggers off several action potentials.
A larger stimulus causes a greater change in membrane voltage, therefore we designate PSP and receptor potential as graded potentials. For example: higher light intensity causes greater hyperpolarization; more Ach causes greater depolarization in neuromuscular junction. Because we do not have fast Na + voltage-dependent channels in the dendrites and body of the neuron, AP cannot be triggered here. The signal propagates electrotonically. It is a redistribution of the charge that has come into the cell in the area of the synapse. Propagation is mainly influenced by the passive properties of the nerve cell membrane; time and length constant .
The distance in which the change in potential falls to 1 / e (representing 37%) of the maximum
value, is called the length or space
constant (\(λ\)). It depends on the electrical properties of the axon and is 0.1-3 mm for the
mammalian axon. Plasmalemma has its own
conductivity and, especially in non-myelinated axons, represents signal loss. The signal thus
depends on the resistance of the
membrane (\(r_m\)) and the resistance of the cytoplasm (\(r_a\)) of the axon and the
resistance of
the extracellular space (\(r_0\)). The
related parameters are captured in the equation:
\[λ = \sqrt{\frac{r_m}{r_a+r_o }}\]
Since the resistance of the cytoplasm, is much higher than the resistance of the extracellular
space, the latter is neglected. Lower
cytoplasmic resistance and higher membrane resistance together increase the length constant. This
means that the action potential will
travel further along the internular segment before the amplitude drops sharply. The myelin sheath
increases the resistance of the
membrane and thus also the length constant. The resistance of the membrane is inversely proportional
to the diameter (larger neurons
therefore have lower resistance), and the resistance of the cytoplasm changes more with the increase
of the neuron, since it is
proportional to \(d^2\) (d = nerve fiber diameter), compared to membrane resistance proportional to
d. Vice versa.
The time constant (\(τ\)) connects the resistance of the membrane and the capacitance of the plasmalemma (\(C_m\)): \[τ = r_m C_m\] Both constants, however, contribute to the speed of translation, which we will see below. hide
The time constant (\(τ\)) connects the resistance of the membrane and the capacitance of the plasmalemma (\(C_m\)): \[τ = r_m C_m\] Both constants, however, contribute to the speed of translation, which we will see below. hide
AXONE HILLOCK - FORMATION OF AP

Many subthreshold potentials are transmitted electrotonically forward over short distances. The electrical impulse therefore dies at a short distance, and does not reach the end of the axon of a large neuron. The membranes of the excitable cells have adapted their membranes
For excitable cells, meaning nerve and muscle cells, is characteristic permeability of their
membrane, which depends
on the type of channels and the number of open channels at a given time. Membranes that allow a
higher flow of ions
at the same concentration gradient are said to be more permeable. Channels are responsible for ion
transport and are
not permeable to all ions equally. Some ion channels are very specific for K + ions, others for Na
+, Ca2 + or for
Cl- ions. Nonspecific cation channels are less specific and leak potassium, sodium, and calcium
ions.
The voltage-dependent channels are opened by a change in the membrane potential (characteristic of Na +, K +, Cl- channels). The tension on the membrane is detected by a specialized part of the transmembrane protein (= ion channel), which changes the configuration of the whole protein. Thus, the channel either opens or closes. Depolarization of the membrane from the resting membrane potential (about - 80 mV) to +20 mV causes the opening of both Na + and the delay of K + channels.
Receptor ion channels are another group of ion channels that require the binding of an appropriate ligand to open (external ligands (e.g. cationic plates in the motor plate), intracellular messengers (e.g. Ca2 + channels in myocardial cells), intracellular metabolites (such as ATP, H +)).
Ion channels can also open due to mechanical stimulation; vibration, pressure (Ca2 + channels in smooth muscle). There are also channels that constantly let ionsin the cell - especially potassium, to a lesser extent sodium - and thus determine the negativity of the cell’s resting membrane potential. hide
The voltage-dependent channels are opened by a change in the membrane potential (characteristic of Na +, K +, Cl- channels). The tension on the membrane is detected by a specialized part of the transmembrane protein (= ion channel), which changes the configuration of the whole protein. Thus, the channel either opens or closes. Depolarization of the membrane from the resting membrane potential (about - 80 mV) to +20 mV causes the opening of both Na + and the delay of K + channels.
Receptor ion channels are another group of ion channels that require the binding of an appropriate ligand to open (external ligands (e.g. cationic plates in the motor plate), intracellular messengers (e.g. Ca2 + channels in myocardial cells), intracellular metabolites (such as ATP, H +)).
Ion channels can also open due to mechanical stimulation; vibration, pressure (Ca2 + channels in smooth muscle). There are also channels that constantly let ionsin the cell - especially potassium, to a lesser extent sodium - and thus determine the negativity of the cell’s resting membrane potential. hide
Sodium channels have the property of refractoriness, which means that they are
inactivated soon
after activation and stop
releasing ions. Inactivation occurs a few ms after activation, so MP does not reach the exact Nerst
sodium potential, but
reaches a peak at about + 20 mV.
The depolarization phase of the action potential lasts 1-2 ms or less because the Na + channels open quickly. Membrane depolarization also opens K + voltage-dependent channels. Nernst's potential for potassium is very negative (-89 mV) and contrasts with very positive sodium. However, because the opening of K + channels happens with a delay, the MP increases. Due to the very negative Nernst potential for potassium and due to the reduced membrane conductivity for sodium and calcium, the AP repolarization phase follows. However, because the K + channels are closing slowly, at some point the MP becomes more negative than the resting membrane potential (MMP) - the hyperpolarization phase.
This refractoriness and inactivation of channels contributes to the inability to reactivate Na + channels regardless of the intensity of the stimulus. The unresponsive state is called the absolute refractory period. The transition from the inactive to the reactive state does not occur in all channels at once, but gradually. Therefore, we cannot provide enough Na + channels for activation during the absolute period.
During the slow closure of K + channels, a larger stimulus is required to trigger a new AP, to activate the Na + voltage dependent channels that removed the inactivation. The period is called the relative refractory period. hide
The depolarization phase of the action potential lasts 1-2 ms or less because the Na + channels open quickly. Membrane depolarization also opens K + voltage-dependent channels. Nernst's potential for potassium is very negative (-89 mV) and contrasts with very positive sodium. However, because the opening of K + channels happens with a delay, the MP increases. Due to the very negative Nernst potential for potassium and due to the reduced membrane conductivity for sodium and calcium, the AP repolarization phase follows. However, because the K + channels are closing slowly, at some point the MP becomes more negative than the resting membrane potential (MMP) - the hyperpolarization phase.
This refractoriness and inactivation of channels contributes to the inability to reactivate Na + channels regardless of the intensity of the stimulus. The unresponsive state is called the absolute refractory period. The transition from the inactive to the reactive state does not occur in all channels at once, but gradually. Therefore, we cannot provide enough Na + channels for activation during the absolute period.
During the slow closure of K + channels, a larger stimulus is required to trigger a new AP, to activate the Na + voltage dependent channels that removed the inactivation. The period is called the relative refractory period. hide
Lidocaine is a local anesthetic. Despite decades of use mechanism of action has not been fully
elucidated. It is said to act on
several biological targets in the body, the most important being the action on the aforementioned Na
+ voltage channels, which
play an essential role in triggering and translating action potentials through nerve and muscle
cells. It specifically blocks
Na + channels in the inactive state, thus preventing the formation of AP. Inhibition of voltage Ca2
+ channels confers an
anesthetic and antiarrhythmic effect (class Ib antiarrhythmic) on lidocaine.
hide
When the signal reaches the axon hillock i.e. the region of the neuron where the density of fast Na + voltage-dependent channels is high, AP can form. Whether the potential change for AP formation will be large enough, depends on the sum of all the effects from different synapses from different neurons on the postsynaptic neuron — that is, all EPSP and IPSP. When the sum rises even more, the AP frequency increases. In this way, the amplitude of the membrane potential is recoded into the frequency of the action potentials.
AXON – SPREAD OF AP
a) AP DOES NOT OCCUR

If the depolarization does not reach the stimulus threshold locally, AP is not provoked at this point. The change of potential depends on the distance, because the magnitude of the change decreases exponentially from the place of origin - the propagation is electrotonic/passive. The changes therefore do not spread far along the axon.
b) AP OCCURS

The basic function of axons is to transfer impulses over longer distances. To illustrate, the axon of the motor nerve is more than 1 meter long. The AP propagates along the entire length of the axon without signal decay. The shape and amplitude of the AP are also constant.
During AP travel, rapid depolarization ensures a sufficiently large flow of ions into the cell. This causes a local change in the MP that propagates electrotonically forward. Local depolarization currents activate Na + voltage-dependent channels and thus depolarize the adjacent part of the membrane. If the threshold is exceeded locally, AP is triggered at this point. The membrane through which the AP has passed becomes transiently refractory, insensitive to new APs. This allows one-way AP translation along the fiber .

Smaller nerve fiber

Bigger nerve fiber

Bigger nerve fiber with myelin sheaths
| Fiber type | Sensory classifitacion | Function | Diameter (mm) | Conduction (m/s) | Local anesthetic activity | Myelinisation |
|---|---|---|---|---|---|---|
| Aα | Motor | 12-20 | 70-120 | + | yes | |
| Aα | type 1a | Prorioception | 12-20 | 70-120 | ++ | yes |
| Aα | type 1b | Prorioception | 12-20 | 70-120 | ++ | yes |
| Aβ | type 2 | Prorioception | 5-12 | 30-70 | ++ | yes |
| Touch pressure | ||||||
| Aδ | type 3 | Pain | 2-5 | 12-30 | +++ | yes |
| Cold temperature | ||||||
| Touch | ||||||
| Aγ | Motor (muscle spindle) | 3-6 | 15-30 | ++ | yes | |
| B | Preganglionic | <3 | 3-14 | ++++ | some | |
| Autonomic fibers | ||||||
| C dorsal root | type 4 | Pain, touch | 0.4-1.2 | 0.5-2 | ++++ | no |
| Warm and cold temperature | ||||||
| C sympathetic | Postganglionic | 0.3-1.3 | 0.7-2.3 | ++++ | no | |
| Sympathetic fibers |
Sphingolipids have fatty acids with an amide bond bound to the alcohol swingosine. Sphingolipids are
composed of myelin,
which surrounds neurite nerve cells like an envelope. Myelin is formed by glia cells; Schwann cells
in the peripheral
nervous system and oligodendrocytes in the central nervous system. The non-polar tails of
sphingosine and fatty acids
are very long, intertwining with each other into a solid structure, causing the myelin sheath to be
very strong. If
myelin synthesis errors occur and the nerve fiber remains “bare,” multiple sclerosis can develop.
Multiple sclerosis
is an autoimmune disease of the central nervous system with various neurological deficits. The
disease mainly affects
women and is characterized by the collapse of myelin sheaths, which leads to muscle weakness,
paralysis, visual disturbances,
paresthesias, etc. Due to the collapse of the myelin sheaths, AP fails to reach Ranvier’s next onset
and AP translation
along the neuron slows down. Neurons are not capable of transmitting impulses.
However, multiple sclerosis is not the only demyelinating disease that can affect humans: Guillain-Barré syndrome is a disease in which autoimmune attacks on peripheral nerves cause damage to the myelin and block the conduction of stimuli through the nerves, leading to muscle paralysis, as well as disturbances in the perception and functioning of the autonomic nervous system. Similarly, in chronic inflammatory demyelinating polyneuropathy, inflammation and autoimmunity lead to demyelination and weakness of the upper and lower limbs. hide
However, multiple sclerosis is not the only demyelinating disease that can affect humans: Guillain-Barré syndrome is a disease in which autoimmune attacks on peripheral nerves cause damage to the myelin and block the conduction of stimuli through the nerves, leading to muscle paralysis, as well as disturbances in the perception and functioning of the autonomic nervous system. Similarly, in chronic inflammatory demyelinating polyneuropathy, inflammation and autoimmunity lead to demyelination and weakness of the upper and lower limbs. hide
TERMINAL PART

As the AP reaches the end it triggers the opening of Ca2 + voltage-dependent channels. Calcium ions enter the cell and bind to a specific protein synaptotagmin, which acts as a calcium sensor and regulates the closure of the SNARE complex (the complex consists of the proteins synaptobrevin, syntaxin and SNAP-25).

Botulinum toxin is a neurotoxic protein produced by the bacterium Clostridium botulinum and related species. It is the cause of botulism. Humans most commonly ingest the toxin from eating improperly canned foods, in which C. botulinum has grown. Toxin can also be introduced through an infected wound. In infants, the bacteria can sometimes grow in the intestines and produce botulinum toxin within the intestine and can cause a condition known as floppy baby syndrome. Toxin binds specifically to nerves which use the neurotransmitter acetylcholine. Once bound to the nerve terminal, the neuron takes up the toxin into a vesicle by receptor-mediated endocytosis. As the vesicle moves farther into the cell, it acidifies, activating a portion of the toxin which triggers it to push across the vesicle membrane and into the cell cytoplasm. Once inside the cytoplasm, the toxin cleaves SNARE proteins, meaning that the acetylcholine vesicles can't bind to the intracellular cell membrane. Preventing the cell from releasing vesicles of neurotransmitter, stops nerve signaling, leading to flaccid paralysis.
In severe cases, the toxin block nerves controlling the respiratory system or heart, resulting in death. Botulism can be difficult to diagnose, as it may appear similar to diseases such as Guillain–Barré syndrome, myasthenia gravis, and stroke. By acting on the muscles, we can relieve muscle cramps or dystonia in cerebral palsy, it can be used to treat certain migraine headaches, and through the effect on the functioning of the sweat glands, we can also treat excessive sweating or hyperhidrosis, etc. Botulin is used in anesthetic medicine to correct facial expressions and smooth wrinkles on the face and neck. The action of botulinum is limited in time to about 4 months, after which it must be re-administered. It may therefore come as no surprise that: The global market for botulinum toxin products, driven by their cosmetic applications, is forecast to reach $ 2.9 billion by 2018. hide

When infected with Clostridium tetani, the tetanus toxin reaches the inhibitory neuron, where it cleaves the synaptobrevin protein from the protein complex, which ensures the fusion of the vesicle with the membrane. Consequently, the fusion of the vesicle containing glycine with the terminal membrane is inhibited. Glycine are not released and cannot pass on the inhibitory effect of the neuron. Because there is no inhibition of contraction, the toxin is also called tetanospasmin, as it causes a characteristic contraction of the body.
The bacteria's toxin reaches the spinal cord via retrograde axonal transport through peripheral nerves or via the blood circulation. Renshaw cells are inhibitory interneurons of the spinal cord that connect to alpha motoneurons of the anterior gray column. They receive input from the descending tracts of supra-spinal origin and the axon collaterals of alpha motoneurons. The neurotransmitter activating Renshaw cells is acetylcholine. Subsequent to their activation, Renshaw cells create an inhibitory postsynaptic potential (IPSP) within the alpha motoneurons by means of inhibitory neurotransmitters glycine and gamma-Aminobutyric acid (GABA). Inhibition prevents overshooting muscle contraction as a result of the muscle's proprioceptive reflex. Alpha motoneurons create a negative feedback loop by causing cholinergic activation of Renshaw cells. This activation of Renshaw cells inhibits the same alpha motoneurons that activated it. This process is called recurrent inhibition. Wound infection with Clostridium tetani causes a pathological disturbance of Renshaw inhibition. Breakdown of Renshaw inhibition first causes hyperreflexia. Later the separate muscular twitches merge into one permanent contraction (spasm) because the alpha motoneurons are activated constantly. This permanent contraction is the reason for the typical signs of tetanus: risus sardonicus, opisthotonus, convulsion. hide
Motor plate is a chemical synapse between the α-motoneuron and the muscle fiber. AP reaching the presynaptic membrane causes follicular exocytosis with the neurotransmitter acetylcholine (ACh). It diffuses to nicotinic acetylcholine receptors on the postsynaptic membrane. Depolarization in the neuromuscular synapse to the excitatory threshold requires 100 "quantans" or vesicles. One vesicle depolarizes the motor plate by 0.4 mV and is said to contain less than 10 000 ACh molecules. Acetylcholinesterase (AChE) is an enzyme, located on the postsynaptic membrane, that is classified as a hydrolase because it hydrolyzes ACh. Each molecule of this enzyme breaks down 25 000 molecules of acetylcholine in one second. The active site of acetylcholinesterase comprises two subsites, the anionic site and the ester site. A positive quaternary amine from the acetylcholine molecule binds to the anionic site but can also bind cationic substrates and inhibitors. The ester site consists of a catalytic triad of three amino acids, namely serine, histidine, and glutamate. At this point, acetylcholine is hydrolyzed to acetate and choline to form the acetyl enzyme and free choline. The acetyl enzyme is subsequently degraded to a free enzyme capable of reactivation and an acetyl group. The newly formed free choline is transferred back to the nerve, where acetylcholine is re-synthesized from acetyl coenzyme A via the action of acetylcholine transferase. Acetylcholine esterase inhibitors are substances that bind to the molecule of the enzyme acetylcholine esterase and thus inhibit the hydrolysis of the natural substrate, i.e. acetylcholine. Inhibitors are either reversible or irreversible. A typical representative of a reversible AChE inhibitor is decamethonium - a derivative of the decamethonium molecule is the drug donepezil, which is used in the treatment of Alzheimer's disease. Other reversible AChE inhibitors used clinically are edrophonium (a short-acting inhibitor used to diagnose myasthenia gravis), neostigmine (a medium-acting inhibitor used orally to treat myasthenia gravis, and parenterally to interrupt neuromuscular block). A medium-acting inhibitor is used in the form of eye drops to treat cataracts (glaucoma). Irreversible AChE inhibitors include insecticides, pesticides, and chemical warfare poisons such as nerve gas.
And the story goes on…
